

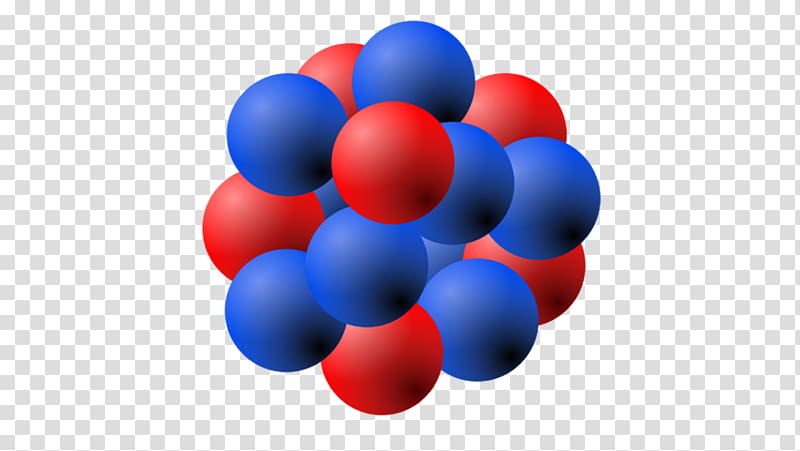
You have already learned that the mass of an electron is very, very small compared to the mass of either a proton or a neutron (like the mass of a penny compared to the mass of a bowling ball). The mass of an atom depends on the number of protons and neutrons. Through the dispersing of alpha particles explored by Rutherford, we discovered that the nucleus of an atom contains a more significant part of the mass of the atom. Why do you think that the "mass number" includes protons and neutrons, but not electrons? You know that most of the mass of an atom is concentrated in its nucleus. The nucleus of an atom is the focal locale of an atom where most of the mass is concentrated. The nuclear strong force extends far enough from each baryon so as to bind the neutrons and protons together against the repulsive electrical force between the positively charged protons. For example, some helium atoms have three neutrons instead of two (these are called isotopes and are discussed in detail later on) The nucleus of an atom consists of neutrons and protons, which in turn are the manifestation of more elementary particles, called quarks, that are held in association by the nuclear strong force in certain stable combinations of hadrons, called baryons. Because the number of neutrons can vary for a given element, the mass numbers of different atoms of an element may also vary. Atoms with the same number of protons but different numbers of neutrons are called isotopes. The nucleus is surrounded by electrons that are negatively charged. In an atom, the nucleus accounts for nearly all of the total mass of the atom.

The bonding of the proton & neutron, proton & proton, neutron & neutron have remained a. An atom is made up of a positively charged, dense, and extremely small nucleus, which contains protons and neutrons, among other elements.
#NUCLEUS ATOM PDF#
However, some helium atoms have more or less than two neutrons. PDF Since the discovery of the atom and nucleus.


 0 kommentar(er)
0 kommentar(er)
